The DermaClip device is a needle-free alternative to sutures, staples and glues for the closure of lacerations, wounds, and surgical incisions.
APPLYING DERMACLIP IS AS SIMPLE AS “PICK, PLACE, PRESS FIRMLY & PULL”
Click below for your preferred materials and method of training:
Full instructions are set out in the Instructions for Use (IFU) that are included with every purchase of DermaClip and can be downloaded here.
Contraindications:


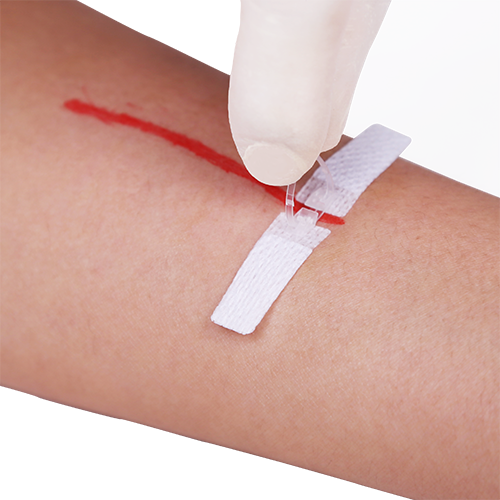
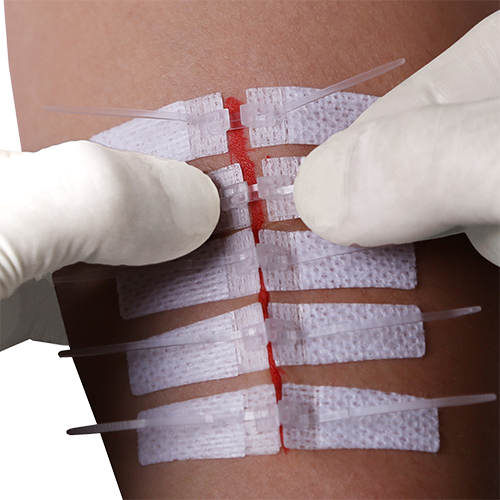
IMPORTANT: There is a first resistance as the device is initially pulled closed that then releases. THIS IS NOT THE LOCKING OF THE DEVICE!
It is very important to the proper functioning of the device that the click occurs after the two edges of the device have made contact and then forced each other, and the attached skin, into an upward position. Only then will the device be closed and locked shut.
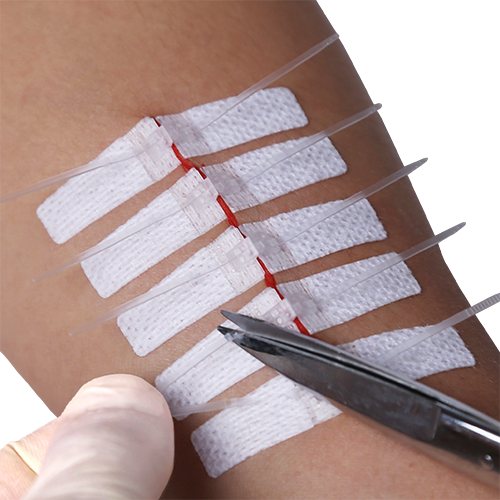
This page and all related content are only meant as a supplement to the full Instructions for Use (IFU).